Care of Client With Fluids and Electrolytes Imbalance (Process of Movement Across The Membranes)
4. MECHANISMS CONTROLLING FLUID AND ELECTROLYTE MOVEMENT
Many different processes are involved in the movement of electrolytes and water between the ICF and ECF. Some of these include:
- simple diffusion
- facilitated diffusion
- active transport
Two forces drive water movement:
- hydrostatic pressure
- osmotic pressure
Osmotic pressure : the amount of pressure required to stop the osmotic flow of water. Measuring osmolality is important because it indicates the water balance of the body.
Isotonic : Fluids with the same osmolality as the cell
Hypotonic : Solutions in which the solutes are less concentrated than the cells
Hypertonic : Solutes more concentrated than cells or an increased osmolality
Hydrostatic pressure : the force of fluid within a compartment and is the major force that pushes water out of the vascular system at the capillary other acts outside the cell.
Fluid Movement
Fluid movement occurs inside the body due to osmotic pressure, hydrostatic pressure, and osmosis. Proper fluid movement depends on intact and properly functioning vascular tissue lining, normal levels of protein content within the blood, and adequate hydrostatic pressures inside the blood vessels. Intact vascular tissue lining prevents fluid from leaking out of the blood vessels. Protein content of the blood (in the form of albumin) causes oncotic pressure that holds water inside the vascular compartment. For example, patients with decreased protein levels (i.e., low serum albumin) experience edema due to the leakage of intravascular fluid into interstitial areas because of decreased oncotic pressure.
Hydrostatic pressure
Hydrostatic pressure is defined as pressure that a contained fluid exerts on what is confining it. In the intravascular fluid compartment, hydrostatic pressure is the pressure exerted by blood against the capillaries. Hydrostatic pressure opposes oncotic pressure at the arterial end of capillaries, where it pushes fluid and solutes out into the interstitial compartment. On the venous end of the capillary, hydrostatic pressure is reduced, which allows oncotic pressure to pull fluids and solutes back into the capillary. See figure below for an illustration of hydrostatic pressure and oncotic pressure in a capillary.
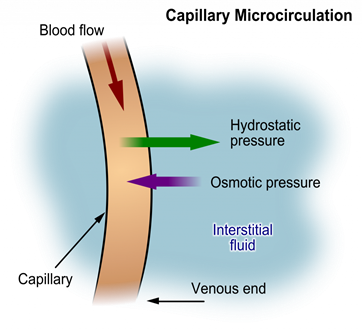
Filtration
Filtration occurs when hydrostatic pressure pushes fluids and solutes through a permeable membrane so they can be excreted. An example of this process is fluid and waste filtration through the glomerular capillaries in the kidneys. This filtration process within the kidneys allows excess fluid and waste products to be excreted from the body in the form of urine.
Fluid movement is also controlled through osmosis. Osmosis is water movement through a semipermeable membrane, from an area of lesser solute concentration to an area of greater solute concentration, in an attempt to equalize the solute concentrations on either side of the membrane. Only fluids and some particles dissolved in the fluid are able to pass through a semipermeable membrane; larger particles are blocked from getting through. Because osmosis causes fluid to travel due to a concentration gradient and no energy is expended during the process, it is referred to as passive transport. See figure below for an illustration of osmosis where water has moved to the right side of the membrane to equalize the concentration of solutes on that side with the left side.
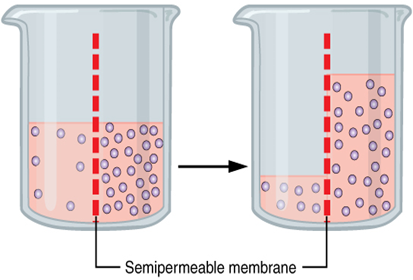
Osmosis causes fluid movement between the intravascular, interstitial, and intracellular fluid compartments based on solute concentration. For example, recall a time when you have eaten a large amount of salty foods. The sodium concentration of the blood becomes elevated. Due to the elevated solute concentration within the bloodstream, osmosis causes fluid to be pulled into the intravascular compartment from the interstitial and intracellular compartments to try to equalize the solute concentration. As fluid leaves the cells, they shrink in size. The shrinkage of cells is what causes many symptoms of dehydration, such as dry, sticky mucous membranes. Because the brain cells are especially susceptible to fluid movement due to osmosis, a headache may occur if adequate fluid intake does not occur.
Solute Movement
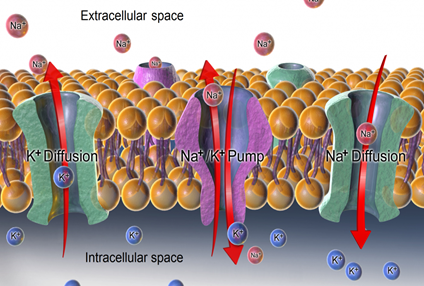
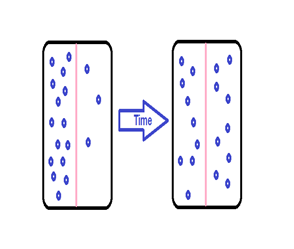
Solute movement is controlled by diffusion, active transport, and filtration. Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration to equalize the concentration of solutes throughout an area. (Note that diffusion is different from osmosis because osmosis is the movement of fluid whereas diffusion is the movement of solutes.) See figure below for an image of diffusion. Because diffusion travels down a concentration gradient, the solutes move freely without energy expenditure. An example of diffusion is the movement of inhaled oxygen molecules from alveoli to the capillaries in the lungs so that they can be distributed throughout the body.
Active transport, unlike diffusion, involves moving solutes and ions across a cell membrane from an area of lower concentration to an area of higher concentration. Because active transport moves solutes against a concentration gradient to prevent an overaccumulation of solutes in an area, energy is required for this process to take place.An example of active transport is the sodium-potassium pump, which uses energy to maintain higher levels of sodium in the extracellular fluid and higher levels of potassium in the intracellular fluid. See Figure below for an image of diffusion and the sodium-potassium pump regulating sodium and potassium levels in the extracellular and intracellular compartments. Recall that sodium (Na+) is the primary electrolyte in the extracellular space and potassium (K+) is the primary electrolyte in the intracellular space.