Care of Client With Fluids and Electrolytes Imbalance (Process of Movement Across The Membranes)
| Site: | Nilai Uni Connect |
| Course: | Perioperative Care; Fluid and Electrolyte |
| Book: | Care of Client With Fluids and Electrolytes Imbalance (Process of Movement Across The Membranes) |
| Printed by: | Guest user |
| Date: | Tuesday, 8 April 2025, 9:56 AM |
1. MECHANISMS CONTROLLING FLUID AND ELECTROLYTE MOVEMENT
Many different processes are involved in the movement of electrolytes and water between the ICF and ECF. Some of the processes include simple diffusion, facilitated diffusion, and active transport. Water moves as driven by two forces: hydrostatic pressure and osmotic pressure.
Osmotic pressure is the amount of pressure required to stop the osmotic flow of water. In the metabolically active cell, there is a constant exchange of substances between the cell and the interstitium, but no net gain or loss of water occurs.
Measuring osmolality is important because it indicates the water balance of the body. Osmolality indicates the concentration of all the particles dissolved in body fluid. It is routinely measured in clinical laboratories for the differential diagnosis of disorders related to the hydrolytic balance regulation, renal function, and small-molecule poisonings.
Hydrostatic pressure is the force within a fluid compartment and is the major force that pushes water out of the vascular system at the capillary level.
Oncotic pressure (colloidal osmotic pressure) is osmotic pressure exerted by colloids in solution. The major colloid in the vascular system contributing to the total osmotic pressure is protein.
2. FLUID MOVEMENT IN CAPILLARIES
The amount and direction of movement between the interstitium and the capillary are determined by the interaction of (1) capillary hydrostatic pressure, (2) plasma oncotic pressure, (3) interstitial hydrostatic pressure, and (4) interstitial oncotic pressure.
If capillary or interstitial pressures are altered, fluid may abnormally shift from one compartment to another, resulting in edema or dehydration.
Fluid is drawn into the plasma space whenever there is an increase in the plasma osmotic or oncotic pressure. This could happen with administration of colloids, dextran, mannitol, or hypertonic solutions.
3. FLUID MOVEMENT BETWEEN EXTRACELLULAR AND INTRACELLULAR FLUID
Changes in the osmolality of the ECF alter the volume of the cells.
Water deficit occurs when an increased ECF osmolality pulls water out of cells until the two compartments have similar osmolality.
Decreased ECF osmolality is associated with water excess as the cells gain excess water.
3.1. What are the mechanisms of controlling fluid and electrolyte movement?
Some of the processes include simple diffusion, facilitated diffusion, and active transport. Water moves as driven by two forces: hydrostatic pressure and osmotic pressure. Osmotic pressure is the amount of pressure required to stop the osmotic flow of water.
The fluid moves between intracellular and extracellular compartments through:
- Osmosis causes fluid movement between the intravascular, interstitial, and intracellular fluid compartments based on solute concentration.
- Movement of water is regulated by controlling the movement of electrolytes between fluid compartments. The movement of water between fluid compartments happens by the process of osmosis.
- The lymphatic system helps maintain fluid balance in the body by collecting excess fluid and particulate matter from tissues and depositing them in the bloodstream. It also helps defend the body against infection by supplying disease-fighting cells called lymphocytes. This article focuses on the human lymphatic.
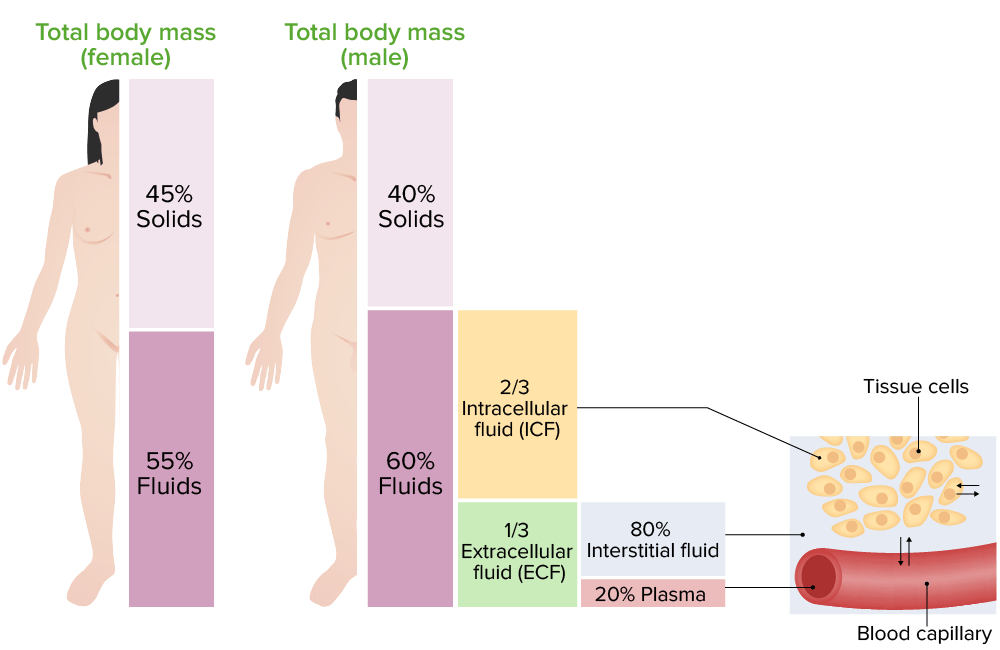
- The intracellular fluid (ICF) and extracellular fluid (ECF) are separated by a semi- permeable cell membrane that is permeable to water but not to most solutes including electrolytes and proteins, which generally need transport systems to move across the membrane.
- In the human body, body fluid is composed of intracellular fluid and extracellular fluid. Movement of water is regulated by controlling the movement of electrolytes between fluid compartments. The movement of water between fluid compartments happens by the process of osmosis.
3.2. How do fluids move between compartment
Fluid Movement between Compartments. Hydrostatic pressure, the force exerted by a fluid against a wall, causes movement of fluid between compartments. The hydrostatic pressure of blood is the pressure exerted by blood against the walls of the blood vessels by the pumping action of the heart.
3.3. How are fluids distributed intracellularly and extracellularly?
Intracellular and extracellular fluids are separated into compartments by semipermeable membranes, and the transport of fluid and ions is maintained by changes.
Water can move freely across the membrane and is directed by the osmotic gradient between the two spaces. Changes in the intracellular fluid volume result from alterations in the osmolarity of the ECF but do not respond to isosmotic changes in extracellular volume Channels in the cell membrane.
Water move between the intracellular and extracellular fluid compartments. When the osmolarity in the ECF rises compared to ICF, water moves by osmosis from the ICF into the ECF
What determines the direction of water movement between fluid compartments?
At the arterial end of a vessel, the hydrostatic pressure is greater than the osmotic pressure, so the net movement favors water and other solutes being passed into the tissue fluid.
At the venous end, the osmotic pressure is greater, so the net movement favors substances being passed back into the capillary. This difference is created by the direction of the flow of blood and the imbalance in solutes created by the net movement of water that favors the tissue fluid.
The kidneys, in concert with neural and endocrine input, regulate the volume and osmolality of the extracellular fluid by altering the amount of sodium and water excreted
A state of balance among all the body systems needed for the body to survive and function correctly. In homeostasis, body levels of acid, blood pressure, blood sugar, electrolytes, energy, hormones, oxygen, proteins, and temperature are constantly adjusted to respond to changes inside and outside the body, to keep them at a normal level.
Within the extracellular fluid, the major cation is sodium and the major anion is chloride. The major cation in the intracellular fluid is potassium. These electrolytes play an important role in maintaining homeostasis.
The main function of intracellular fluid is to help with the transport of gases, nutrients, and other molecules. Intracellular fluid is also important for intracellular communication and cell signaling. Extracellular fluid is found in blood plasma and in the interstitial space between cells.
3.4. What electrolyte regulates extracellular fluid volume?
Sodium
Sodium, which is an osmotically active cation, is one of the most important electrolytes in the extracellular fluid. It is responsible for maintaining the extracellular fluid volume, and also for regulation of the membrane potential of cells.
What electrolytes are intracellular?
In the intracellular fluid, K+ and HPO4- are the major electrolytes. also of crucial importance in regulating fluid balance in the body. Sodium levels are extremely closely regulated by kidney function. most of it is reabsorbed in the kidney tubules.
What is the difference between intracellular and extracellular?
The significant difference between intracellular and extracellular is that one acts inside the cell, and the other acts outside the cell.
4. MECHANISMS CONTROLLING FLUID AND ELECTROLYTE MOVEMENT
Many different processes are involved in the movement of electrolytes and water between the ICF and ECF. Some of these include:
- simple diffusion
- facilitated diffusion
- active transport
Two forces drive water movement:
- hydrostatic pressure
- osmotic pressure
Osmotic pressure : the amount of pressure required to stop the osmotic flow of water. Measuring osmolality is important because it indicates the water balance of the body.
Isotonic : Fluids with the same osmolality as the cell
Hypotonic : Solutions in which the solutes are less concentrated than the cells
Hypertonic : Solutes more concentrated than cells or an increased osmolality
Hydrostatic pressure : the force of fluid within a compartment and is the major force that pushes water out of the vascular system at the capillary other acts outside the cell.
Fluid Movement
Fluid movement occurs inside the body due to osmotic pressure, hydrostatic pressure, and osmosis. Proper fluid movement depends on intact and properly functioning vascular tissue lining, normal levels of protein content within the blood, and adequate hydrostatic pressures inside the blood vessels. Intact vascular tissue lining prevents fluid from leaking out of the blood vessels. Protein content of the blood (in the form of albumin) causes oncotic pressure that holds water inside the vascular compartment. For example, patients with decreased protein levels (i.e., low serum albumin) experience edema due to the leakage of intravascular fluid into interstitial areas because of decreased oncotic pressure.
Hydrostatic pressure
Hydrostatic pressure is defined as pressure that a contained fluid exerts on what is confining it. In the intravascular fluid compartment, hydrostatic pressure is the pressure exerted by blood against the capillaries. Hydrostatic pressure opposes oncotic pressure at the arterial end of capillaries, where it pushes fluid and solutes out into the interstitial compartment. On the venous end of the capillary, hydrostatic pressure is reduced, which allows oncotic pressure to pull fluids and solutes back into the capillary. See figure below for an illustration of hydrostatic pressure and oncotic pressure in a capillary.
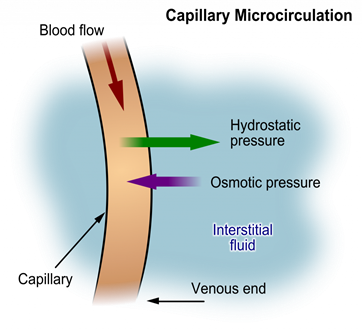
Filtration
Filtration occurs when hydrostatic pressure pushes fluids and solutes through a permeable membrane so they can be excreted. An example of this process is fluid and waste filtration through the glomerular capillaries in the kidneys. This filtration process within the kidneys allows excess fluid and waste products to be excreted from the body in the form of urine.
Fluid movement is also controlled through osmosis. Osmosis is water movement through a semipermeable membrane, from an area of lesser solute concentration to an area of greater solute concentration, in an attempt to equalize the solute concentrations on either side of the membrane. Only fluids and some particles dissolved in the fluid are able to pass through a semipermeable membrane; larger particles are blocked from getting through. Because osmosis causes fluid to travel due to a concentration gradient and no energy is expended during the process, it is referred to as passive transport. See figure below for an illustration of osmosis where water has moved to the right side of the membrane to equalize the concentration of solutes on that side with the left side.
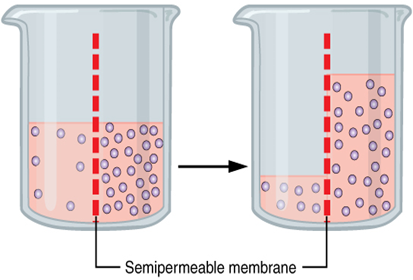
Osmosis causes fluid movement between the intravascular, interstitial, and intracellular fluid compartments based on solute concentration. For example, recall a time when you have eaten a large amount of salty foods. The sodium concentration of the blood becomes elevated. Due to the elevated solute concentration within the bloodstream, osmosis causes fluid to be pulled into the intravascular compartment from the interstitial and intracellular compartments to try to equalize the solute concentration. As fluid leaves the cells, they shrink in size. The shrinkage of cells is what causes many symptoms of dehydration, such as dry, sticky mucous membranes. Because the brain cells are especially susceptible to fluid movement due to osmosis, a headache may occur if adequate fluid intake does not occur.
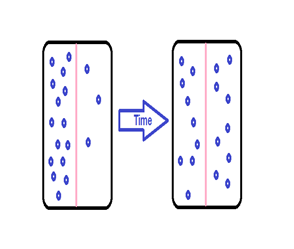
Solute Movement
Solute movement is controlled by diffusion, active transport, and filtration. Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration to equalize the concentration of solutes throughout an area. (Note that diffusion is different from osmosis because osmosis is the movement of fluid whereas diffusion is the movement of solutes.) See figure below for an image of diffusion. Because diffusion travels down a concentration gradient, the solutes move freely without energy expenditure. An example of diffusion is the movement of inhaled oxygen molecules from alveoli to the capillaries in the lungs so that they can be distributed throughout the body.
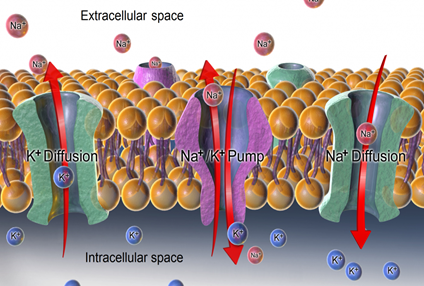
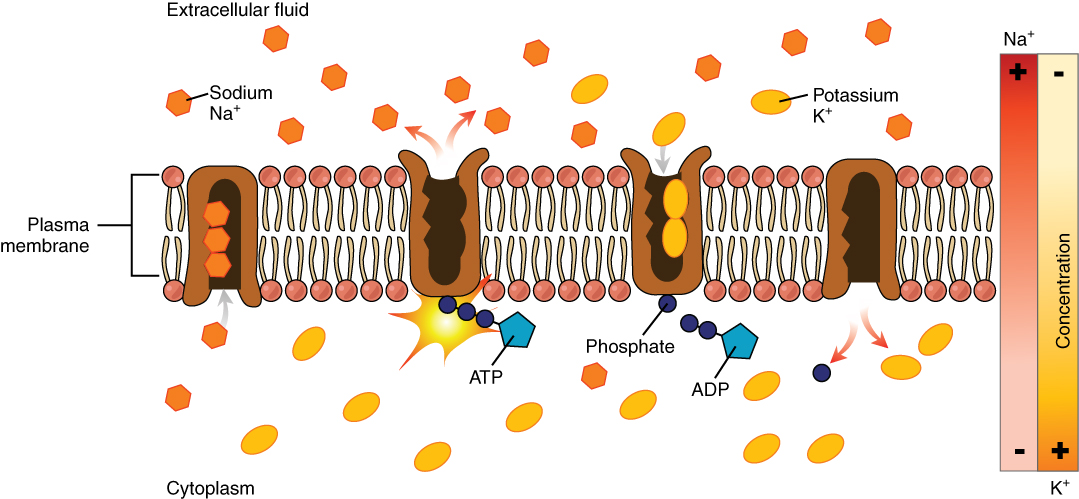
Active transport, unlike diffusion, involves moving solutes and ions across a cell membrane from an area of lower concentration to an area of higher concentration. Because active transport moves solutes against a concentration gradient to prevent an overaccumulation of solutes in an area, energy is required for this process to take place.An example of active transport is the sodium-potassium pump, which uses energy to maintain higher levels of sodium in the extracellular fluid and higher levels of potassium in the intracellular fluid. See Figure below for an image of diffusion and the sodium-potassium pump regulating sodium and potassium levels in the extracellular and intracellular compartments. Recall that sodium (Na+) is the primary electrolyte in the extracellular space and potassium (K+) is the primary electrolyte in the intracellular space.