Topic 14: Acids and Bases
TLO: Compare the role of buffers, exhalation of carbon dioxide, and kidney excretion of H+ in maintaining pH of body fluids
To achieve acid-base balance, there must be a balance between the intake or production of hydrogen ions and net removal of hydrogen ions from the body. The various mechanisms that contribute to the regulation of hydrogen ion concentration are discussed in this chapter.
ACIDS, BASES AND BUFFERS
An acid is defined as a substance that releases protons or hydrogen ions (H+), e.g. hydrochloric acid (HCI), carbonic acid (H2CO3). While a base is a substance that accepts protons or hydrogen ions, e.g. bicarbonate ion (HCO3 –), and HPO42- Proteins in the body also function as bases, because some of the amino acids accept hydrogen ions, e.g. haemoglobin in red blood cells and plasma protein especially albumin is the most important of the body’s bases.
Buffer is a solution of weak acid and the salt of that acid (which functions as weak base) which resists a change in pH when a small amount of acid or base is added to it. By buffering mechanism, a strong acid (or base) is replaced by a weaker one.
NORMAL pH OF THE BODY FLUIDS
The normal pH of arterial blood is 7.4, whereas the pH of venous blood and interstitial fluids is about 7.35 because of the extra amounts of carbon dioxide (CO2), released from the tissues to form H2CO3 in these fluids. Thus, the pH of blood is maintained within a remarkable constant level of 7.35–7.45.
The maintenance of a constant pH is important because, the activities of almost all enzyme systems in the body are influenced by hydrogen ion concentration. Therefore, changes in hydrogen ion concentration alter virtually all cell and body functions, the conformation of biological structural components and uptake and release of oxygen.
REGULATION OF BLOOD pH
To maintain the blood pH at 7.35 –7.45, there are three primary systems that regulate the hydrogen ion concentration in the body fluids. These are:
- Buffer mechanism: First line of defence.
- The respiratory mechanism: Second line of defence.
- Renal mechanism: Third line of defence.
The first two lines of defence keep the hydrogen ion concentration from changing too much until the more slowly responding third line of defence, the kidneys, can eliminate the excess acid or base from the body.
Buffer systems and their role in acid-base balance
The buffer systems of the blood, tissue fluids and cells; immediately combine with acid or base to prevent excessive changes in hydrogen ion concentration. Buffer systems do not eliminate hydrogen ions from the body or add them to the body but only keep them tied up until balance can be re-established.
Various buffer systems are present within the body as stated in the diagram below:
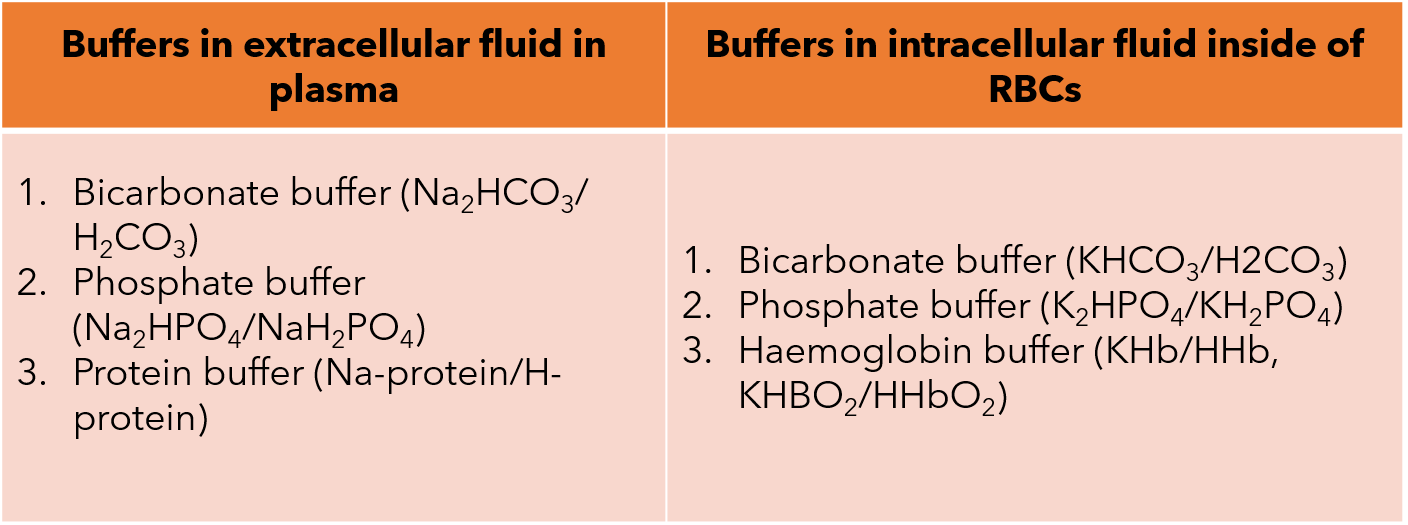
The buffer systems can also be classified according to the location; either extracellularly or intracellularly. The systems are mentioned in the table below: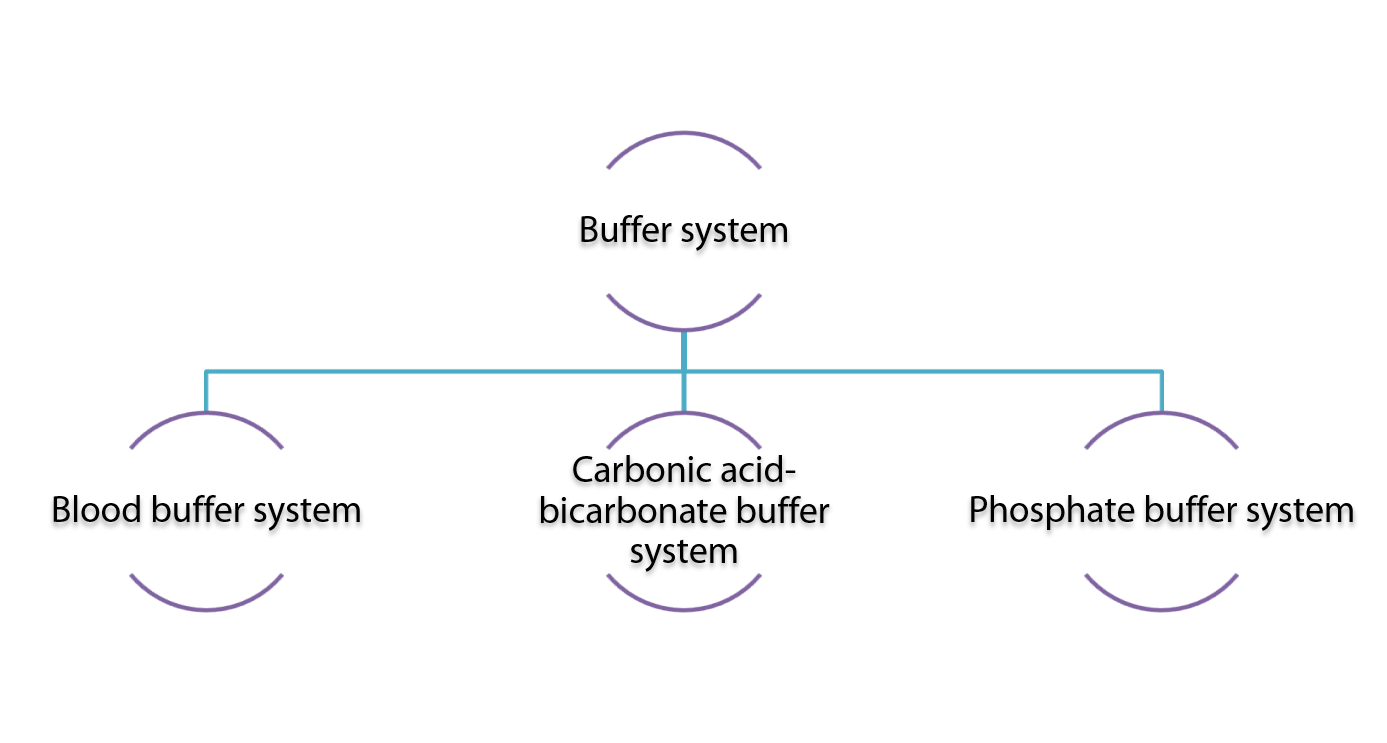
- The bicarbonate buffer system (HCO3-/ H2CO3)
The bicarbonate buffer system is the most important extracellular buffer. The mechanism of action for the bicarbonate buffer system includes:
When a strong acid, such as HCI, is added to the bicarbonate buffer solution, the increased hydrogen ions are buffered by HCO3–.
This step is formulated as so; HCO3- + H+ à H2CO3.
Thus, hydrogen ions from strong acid (HCI) react with HCO3 to form very weak acid H2CO3.
The opposite reactions take place when a strong base such as sodium hydroxide (NaOH), is added to the bicarbonate buffer solution.
This step is formulated likewise; NaOH +H2CO3 à NaHCO3 +H2O.
In this case, the hydroxyl ion (OH–) from NaOH combines with H2CO3 to form weak base HCO3–. Thus, strong base NaOH is replaced by a weak base NaHCO3.
2. The phosphate buffer system (HPO42- / H2PO4-)
The phosphate buffer system is not important as a blood buffer, it plays a major role in buffering renal tubular fluid and intracellular fluids. Its concentration in both plasma and erythrocytes is low, i.e. only 8% of the concentration of the bicarbonate buffer. Therefore, the total buffering power of the phosphate system in the blood is much less than that of the bicarbonate buffering system.
The mechanism of action for phosphate buffer are:
When a strong acid like HCl is added to the phosphate buffer system, the H+ is accepted by the base HPO42- and converted to H2PO4- which then minimize the pH reduction. The step is formulated likewise;
HCl + Na2HPO4 à H2PO4- + NaCl
When a strong base like NaOH is added to the phosphate buffer system, the OH- is buffered by the H2PO4- to form HPO42- and water. Thus, strong base NaOH is replaced by weak base HPO42- leading to increase of pH. The step is formulated as so;
NaOH + NaH2PO4- à Na2HPO4 +H2O
3. Protein buffer
i. Plasma protein buffer: In the blood, plasma proteins especially albumin act as buffer because proteins contain a large number of dissociable acidic (COOH) and basic (NH2) groups in their structure. In acid solution they act as a buffer in that, the basic amino group (NH2) takes up excess H+ ions forming (NH3+). Whereas in basic solutions the acidic COOH groups give up hydrogen ion forming OH– of alkali to water.
ii. Haemoglobin buffer: Haemoglobin is the major intracellular buffer of blood which is present in erythrocytes. It buffers carbonic acid (H2CO3) and its anhydride CO2 from the tissues.